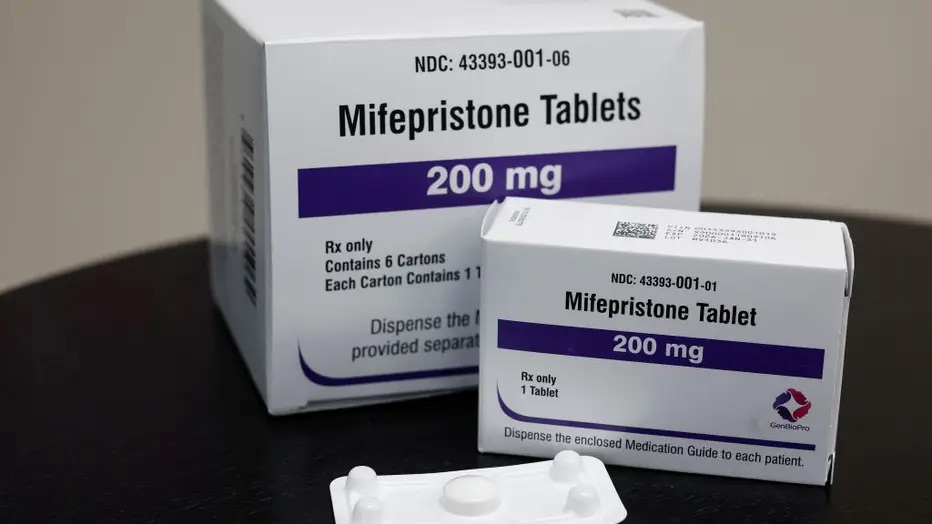
Photo courtesy of Fox 4 News
The debate over access to mifepristone, a widely used abortion medication, has intensified after a Texas federal judge allowed Idaho, Kansas, and Missouri to challenge existing federal rules. These states aim to impose stricter regulations on the drug, including banning telehealth prescriptions and mandating in-person visits for dispensing.
This ruling, handed down just days before President-elect Donald Trump takes office, has the potential to reshape access to medication abortion, now the most common method of terminating pregnancies in the United States.
What Does the Texas Ruling Mean?
Background:
U.S. District Judge Matthew Kacsmaryk, a Trump appointee known for previous rulings against the Biden administration, determined that the states’ arguments deserve further examination. The states claim that the FDA’s relaxed regulations—such as allowing telehealth prescriptions—conflict with their abortion laws and hinder enforcement efforts.
This legal battle follows a U.S. Supreme Court decision last year, which preserved access to mifepristone by dismissing earlier challenges due to lack of standing.
Why Is Mifepristone a Target?
Broader Context:
Since the Supreme Court overturned Roe v. Wade, opponents of abortion have increasingly focused on restricting medication abortion. Mifepristone, often used in combination with a second drug, now accounts for over 60% of abortions in the U.S.
States like Idaho and Kansas, which have differing abortion restrictions, are united in this legal push to tighten control over the drug. In contrast, Democratic-led states have worked to protect access, implementing measures such as allowing telehealth prescriptions and safeguarding providers from out-of-state investigations.
What’s Next for Mifepristone Access?
Future Implications:
The Texas court’s decision permits Idaho, Kansas, and Missouri to challenge the FDA’s mifepristone regulations, with Judge Kacsmaryk presiding over the case. Legal experts warn that the outcome could have far-reaching effects, not only for medication abortion but also for the FDA’s regulatory authority more broadly.
As the case progresses, states with restrictive abortion laws are closely monitoring developments. Any rollback of FDA rules could embolden their efforts to further limit abortion access.