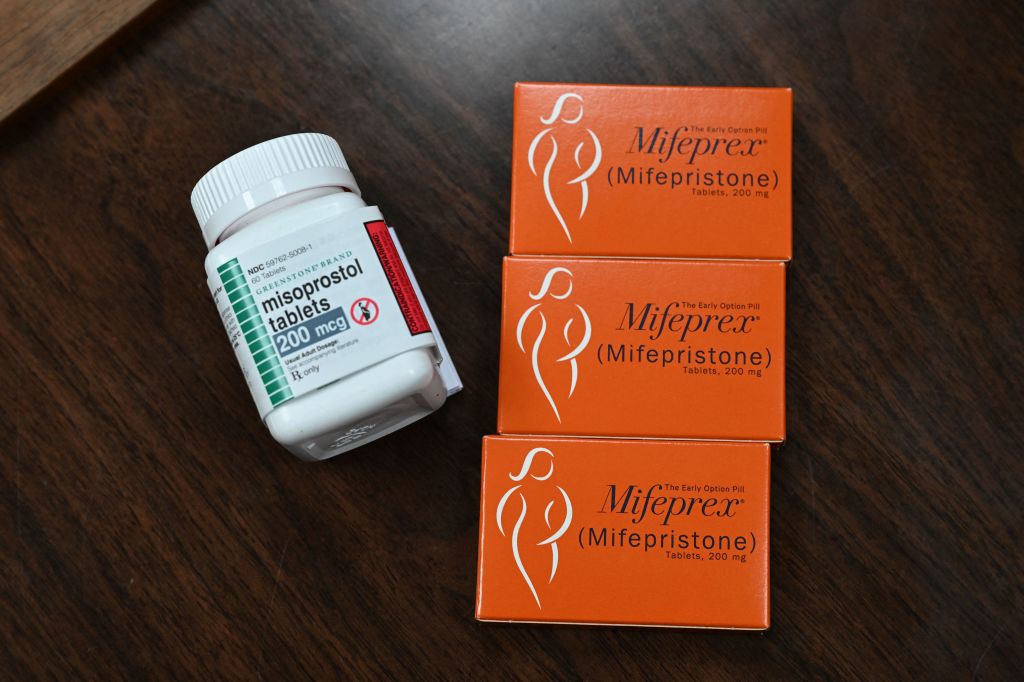
WASHINGTON (AP) — The U.S. Supreme Court on Thursday ruled unanimously to preserve access to the abortion pill mifepristone, a pill used in the most common way to end a pregnancy. The medication was used in nearly two-thirds of all abortions in the United States last year.
The ruling is the court’s first abortion decision since conservative justices overturned Roe v. Wade two years ago.
The justices ruled that abortion opponents lacked the legal right to sue over the federal Food and Drug Administration’s approval of the medication, mifepristone, and the FDA’s subsequent actions to ease access to it.
Justice Brett Kavanaugh wrote for the court that “federal courts are the wrong forum for addressing the plaintiffs’ concerns about FDA’s actions.” Kavanaugh was part of the majority to overturn Roe.
The case had threatened to restrict access to mifepristone across the country, including in states where abortion remains legal.
How safe is mifepristone?
When the U.S. Supreme Court heard oral arguments for the case in March, the safety of mifepristone was at the heart of the debate.
There are rare occasions when mifepristone can cause dangerous, excessive bleeding that requires emergency care. Because of that, the Food and Drug Administration imposed strict safety limits on who could prescribe and distribute it.
The doctors also had to be capable of performing emergency surgery to stop excess bleeding and an abortion procedure if the drug didn’t end the pregnancy. Over the years, the FDA reaffirmed mifepristone’s safety and repeatedly eased restrictions, culminating in a 2021 decision doing away with any in-person requirements and allowing the pill to be sent through the mail.
Abortion rights groups respond to abortion pill ruling
Nancy Northup, president and CEO of the Center for Reproductive Rights, expressed relief at Thursday’s decision by the U.S. Supreme Court on access to mifepristone but also expressed frustration that the case made it the court at all, calling it “meritless.”
“Unfortunately, the attacks on abortion pills will not stop here — the anti-abortion movement sees how critical abortion pills are in this post-Roe world, and they are hell-bent on cutting off access,” she added.
Mini Timmaraju, president and CEO of the national abortion rights group Reproductive Freedom for All, echoed similar feelings. While expressing relief she also said, “This baseless push to block abortion access should never have been heard by them in the first place.”
How the case ended up at the US Supreme Court
The mifepristone case began five months after the Supreme Court overturned Roe v. Wade. Abortion opponents initially won a sweeping ruling nearly a year ago from U.S. District Judge Matthew Kacsmaryk, a Trump nominee in Texas, that would have revoked the drug’s approval entirely.
The 5th U.S. Circuit Court of Appeals left intact the FDA’s initial approval of mifepristone, but it would reverse changes regulators made in 2016 and 2021 that eased some conditions for administering the drug.
The Supreme Court put the appeals court’s modified ruling on hold and agreed to hear the case. However, Justices Samuel Alito — the author of the decision overturning Roe — and Clarence Thomas would have allowed some restrictions to take effect while the case proceeded.
Here’s what each side argued in the case
Health care providers have said that if mifepristone is no longer available or is too hard to obtain, they would switch to using only misoprostol, which is somewhat less effective in ending pregnancies.
U.S. President Joe Biden’s administration and drug manufacturers had warned that siding with abortion opponents in the case could undermine the FDA’s drug approval process beyond the abortion context by inviting judges to second-guess the agency’s scientific judgments. The Democratic administration and New York-based Danco Laboratories, which makes mifepristone, argued that the drug is among the safest the FDA has ever approved.
The abortion opponents argued in court papers that the FDA’s decisions in 2016 and 2021 to relax restrictions on getting the drug were unreasonable and “jeopardize women’s health across the nation.”
What is mifepristone?
More than 6 million people have used mifepristone since 2000. Mifepristone blocks the hormone progesterone and primes the uterus to respond to the contraction-causing effect of a second drug, misoprostol. The two-drug regimen has been used to end a pregnancy through 10 weeks gestation.
US Supreme Court rules to preserve access to abortion medication
The Supreme Court on Thursday unanimously preserved access to a medication that was used in nearly two-thirds of all abortions in the U.S. last year, in the court’s first abortion decision since conservative justices overturned Roe v. Wade two years ago.
The justices ruled that abortion opponents lacked the legal right to sue over the federal Food and Drug Administration’s approval of the medication, mifepristone, and the FDA’s subsequent actions to ease access to it.
The case had threatened to restrict access to mifepristone across the country, including in states where abortion remains legal.
Copyright 2023. Associated Press. All Rights Reserved.