Silver Spring, MD (WBAP/KLIF News ) – The U.S. Food and Drug Administration approved the first over-the-counter birth control pill.
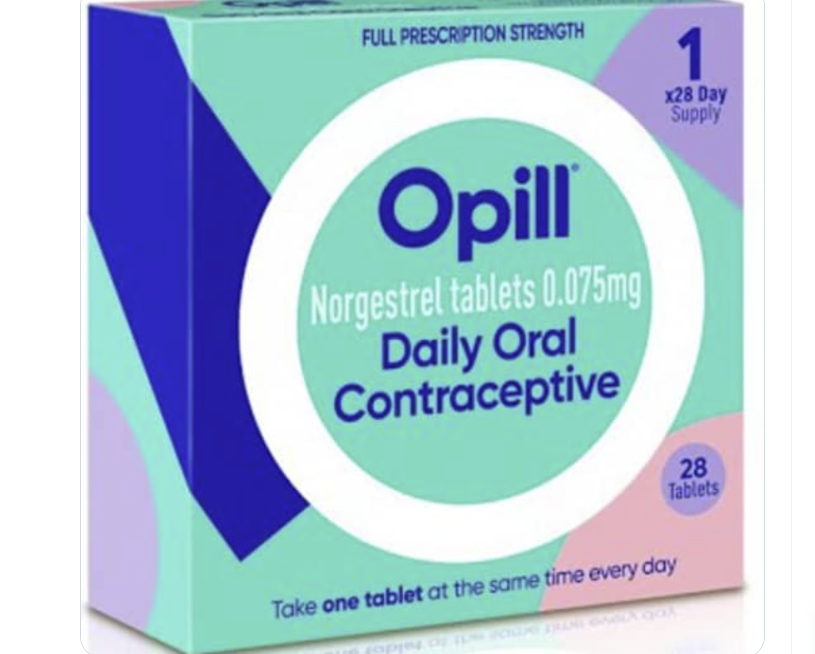
Thursday’s approval of the oral contraceptive Opill will allow consumers to purchase a birth control pill without a prescription in the U.S.
The tablet only contains progestin and is taken once daily. It can be purchased at drug stores, convenience stores, grocery stores and online.
The FDA granted to approval to Laboratoire HRA Pharma, which was recently bought by Perrigo Company plc.
“Today’s approval marks the first time a nonprescription daily oral contraceptive will be an available option for millions of people in the United States,” said Patrizia Cavazzoni, M.D., director of the FDA’s Center for Drug Evaluation and Research. “When used as directed, daily oral contraception is safe and is expected to be more effective than currently available nonprescription contraceptive methods in preventing unintended pregnancy.”
According to the FDA, almost half of the 6.1 million pregnancies in the U.S. each year are unintended. Unintended pregnancies have been linked to negative maternal and perinatal outcomes, including reducing the likelihood of receiving early prenatal care and increased risk of preterm delivery, which could lead to adverse neonatal, developmental and child health outcomes.
The FDA said Opill’s availability may help reduce the number of unintended pregnancies and their negative potential impacts.
The development is considered a massive when for medical groups, including the American Medical Association, which have long fought for an over-the -counter birth control pill.
It also comes as legal battles over women’s reproductive rights, including here in Texas, are being fought nationwide.
Advocacy groups reacted to the news on social media:
Click here for more information about the Opill.
Copyright 2023. WBAP/KLIF News. All Rights Reserved.